A Smart Approach:
TrelliX Embolic Coil
More volume, less metal
TRELLIX
TrelliX Embolic Coil
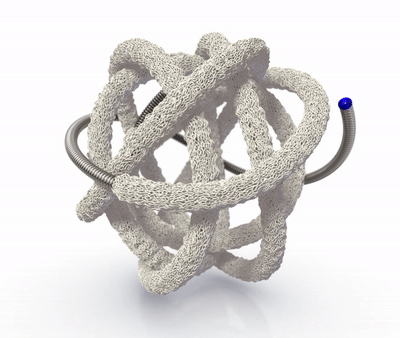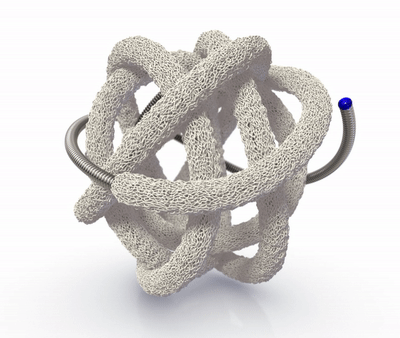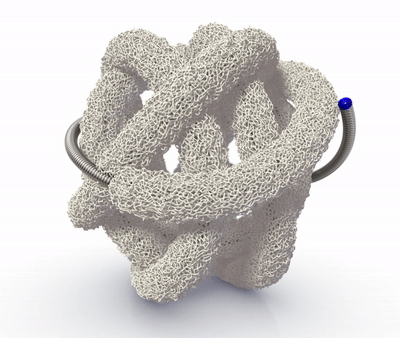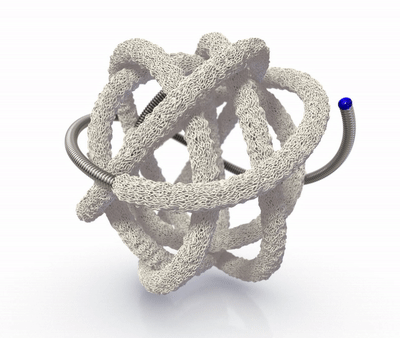
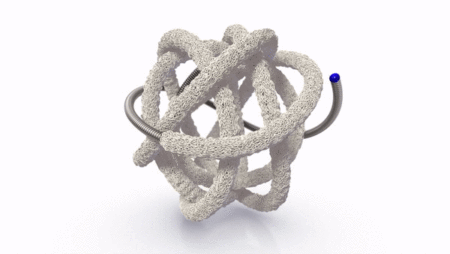
The TrelliX Embolic Coil comprises shape memory polymer over a coil. The polymer is crimped over a platinum-tungsten coil for catheter delivery and self-expands to a predefined volume upon contact with blood.
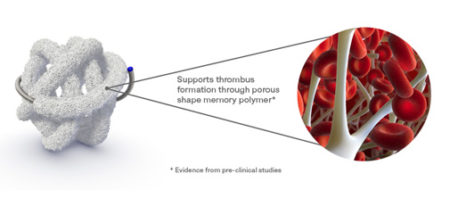
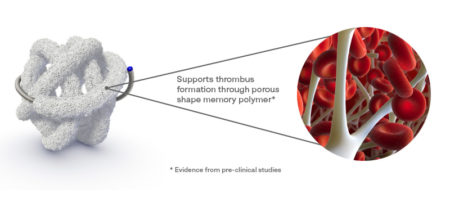
High-volume, porous smart polymer promotes rapid clot formation, stable occlusion, and durable seal.
Regenerative smart polymer stimulates the immune response and healing process to promote healthy new cellular growth as the material slowly bioabsorbs without chronic inflammation. *
Radiolucent smart material restores unprecedented visibility during and after the procedure.
*Evidence from pre-clinical studies
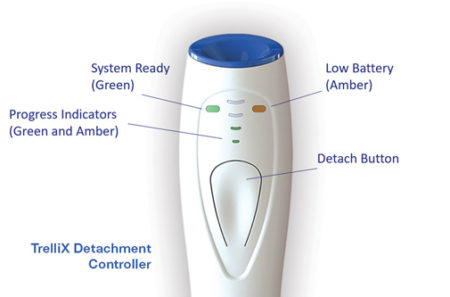
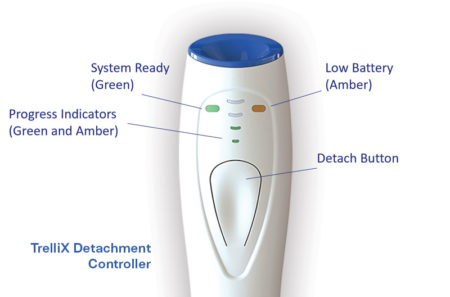
TrelliX Detachment Controller
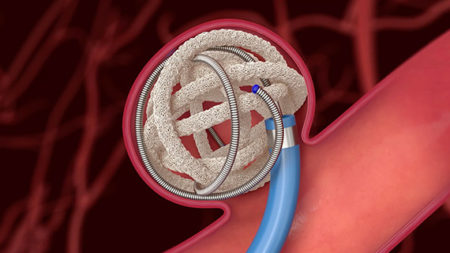
TrelliX Embolic Coil in action
INDICATIONS (Countries that recognize CE marking) – The TrelliX Embolic Coil System is intended to obstruct or occlude blood flow in vascular abnormalities of the neurovascular and peripheral vessels. Indications include intracranial aneurysms, other neurovascular abnormalities such as arteriovenous malformations and arteriovenous fistulae, and arterial and venous embolizations in the peripheral vasculature.
The TrelliX Detachment Controller is intended for use with the TrelliX Embolic Coil System which is intended to obstruct or occlude blood flow in vascular abnormalities of the neurovascular and peripheral vessels. Indications include intracranial aneurysms, other neurovascular abnormalities such as arteriovenous malformations and arteriovenous fistulae, and arterial and venous embolizations in the peripheral vasculature.
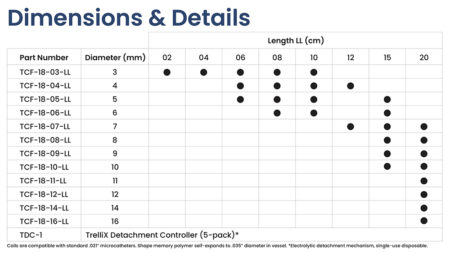
Market Approvals: The TrelliX Embolic Coil has received CE Mark. TrelliX is not approved for sale in the United States or Japan. Find your local distributor.
Indications, contraindications, warnings, and instructions for use can be found in the product labeling supplied with each device. See all Indications, Safety & Warnings
TRELLIX
TrelliX Embolic Coil
The TrelliX Embolic Coil comprises shape memory polymer over a coil. The polymer is crimped over a platinum-tungsten coil for catheter delivery and self-expands to a predefined volume upon contact with blood.
High-volume, porous smart polymer promotes rapid clot formation, stable occlusion, and durable seal.
Regenerative smart polymer stimulates the immune response and healing process to promote healthy new cellular growth as the material slowly bioabsorbs without chronic inflammation. *
Radiolucent smart material restores unprecedented visibility during and after the procedure.
*Evidence from pre-clinical studies
TrelliX Detachment Controller
TrelliX Embolic Coil in action

INDICATIONS (Coountries that recognize CE marking) – The TrelliX Embolic Coil System is intended to obstruct or occlude blood flow in vascular abnormalities of the neurovascular and peripheral vessels. Indications include intracranial aneurysms, other neurovascular abnormalities such as arteriovenous malformations and arteriovenous fistulae, and arterial and venous embolizations in the peripheral vasculature.
The TrelliX Detachment Controller is intended for use with the TrelliX Embolic Coil System which is intended to obstruct or occlude blood flow in vascular abnormalities of the neurovascular and peripheral vessels. Indications include intracranial aneurysms, other neurovascular abnormalities such as arteriovenous malformations and arteriovenous fistulae, and arterial and venous embolizations in the peripheral vasculature.
Market Approvals: The TrelliX Embolic Coil has received CE Mark. TrelliX is not approved for sale in the United States or Japan. Find your local distributor.
Indications, contraindications, warnings, and instructions for use can be found in the product labeling supplied with each device. See all Indications, Safety & Warnings
TRELLIX
TrelliX Embolic Coil
The TrelliX Embolic Coil comprises shape memory polymer over a coil. The polymer is crimped over a platinum-tungsten coil for catheter delivery and self-expands to a predefined volume upon contact with blood.
High-volume, porous smart polymer promotes rapid clot formation, stable occlusion, and durable seal.
Regenerative smart polymer stimulates the immune response and healing process to promote healthy new cellular growth as the material slowly bioabsorbs without chronic inflammation. *
Radiolucent smart material restores unprecedented visibility during and after the procedure.
*Evidence from pre-clinical studies
TrelliX Detachment Controller
TrelliX Embolic Coil in action

INDICATIONS (Coountries that recognize CE marking) – The TrelliX Embolic Coil System is intended to obstruct or occlude blood flow in vascular abnormalities of the neurovascular and peripheral vessels. Indications include intracranial aneurysms, other neurovascular abnormalities such as arteriovenous malformations and arteriovenous fistulae, and arterial and venous embolizations in the peripheral vasculature.
The TrelliX Detachment Controller is intended for use with the TrelliX Embolic Coil System which is intended to obstruct or occlude blood flow in vascular abnormalities of the neurovascular and peripheral vessels. Indications include intracranial aneurysms, other neurovascular abnormalities such as arteriovenous malformations and arteriovenous fistulae, and arterial and venous embolizations in the peripheral vasculature.
Market Approvals: The TrelliX Embolic Coil has received CE Mark. TrelliX is not approved for sale in the United States or Japan. Find your local distributor.
Indications, contraindications, warnings, and instructions for use can be found in the product labeling supplied with each device. See all Indications, Safety & Warnings