A Smart Approach:
IMPEDE Embolization Plug Family
Generates new healing possibilities – Conforms to the anatomy – Returns clarity – Delivers unmatched volume
Success with Less
IMPEDE PRODUCT FAMILY
IMPEDE Embolization Plug
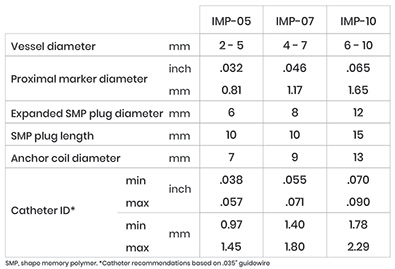
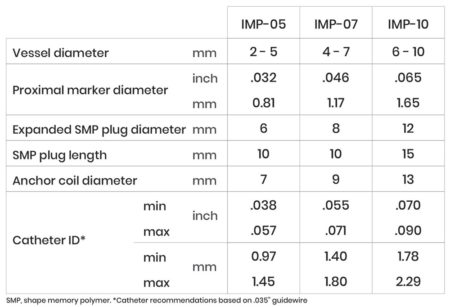
The IMPEDE Embolization Plug consists of the novel smart polymer and an anchor coil for device stability. With a single device, IMPEDE fills the space predictably and effectively. The device requires minimal delivery force and conforms to the anatomy without vessel distortion.
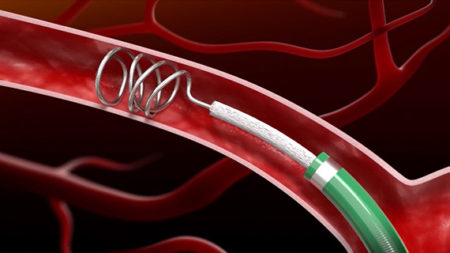

INDICATIONS – The IMPEDE Embolization Plug is indicated to obstruct or reduce the rate of blood flow in the peripheral vasculature.
Market Approvals: The IMPEDE Embolization Plug is FDA cleared, CE marked, and PMDA approved. Find your local distributor.
Federal (USA) law restricts these devices to sale, distribution, and use by or on the order of a physician. Indications, contraindications, warnings, and instructions for use can be found in the product labeling supplied with each device. See all Indications, Safety & Warnings
IMPEDE PRODUCT FAMILY
IMPEDE Embolization Plug
The IMPEDE Embolization Plug consists of the novel smart polymer and an anchor coil for device stability. With a single device, IMPEDE fills the space predictably and effectively. The device requires minimal delivery force and conforms to the anatomy without vessel distortion.


INDICATIONS – The IMPEDE Embolization Plug is indicated to obstruct or reduce the rate of blood flow in the peripheral vasculature.
Market Approvals: The IMPEDE Embolization Plug is FDA cleared, CE marked, and PMDA approved. Find your local distributor.
Federal (USA) law restricts these devices to sale, distribution, and use by or on the order of a physician. Indications, contraindications, warnings, and instructions for use can be found in the product labeling supplied with each device. See all Indications, Safety & Warnings
IMPEDE PRODUCT FAMILY
IMPEDE Embolization Plug
The IMPEDE Embolization Plug consists of the novel smart polymer and an anchor coil for device stability. With a single device, IMPEDE fills the space predictably and effectively. The device requires minimal delivery force and conforms to the anatomy without vessel distortion.


INDICATIONS – The IMPEDE Embolization Plug is indicated to obstruct or reduce the rate of blood flow in the peripheral vasculature.
Market Approvals: The IMPEDE Embolization Plug is FDA cleared, CE marked, and PMDA approved. Find your local distributor.
Federal (USA) law restricts these devices to sale, distribution, and use by or on the order of a physician. Indications, contraindications, warnings, and instructions for use can be found in the product labeling supplied with each device. See all Indications, Safety & Warnings